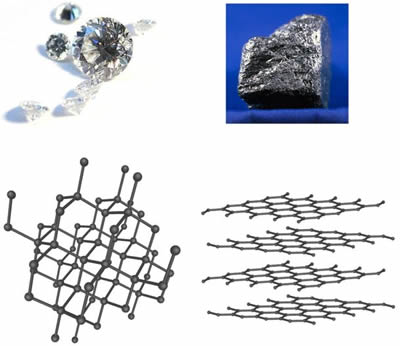
Graphite ˈ ɡ r æ f aɪ t archaically referred to as plumbago is a crystalline form of the element carbon with its atoms arranged in a hexagonal structure it occurs naturally in this form and is the most stable form of carbon under standard conditions under high pressures and temperatures it converts to diamond graphite is used in pencils and lubricants.
Uses of diamond and graphite class 10.
Can form families of.
In this video we explore the diamond and graphite which are two allotropes of solid carbon and we compare their structure and properties.
Unlike diamond graphite can be used as a lubricant or in pencils because the layers cleave readily.
Each carbon atom can form four covalent bonds.
Graphite can be used to make graphene sheets.
Graphite also has.
Graphene a naturally occurring ingredient in graphite has unique physical properties and is one of the strongest known substances.
This is just a demo video for more videos and full syllabus please contact us on 8287971571 or 0261 4890014.
4 iupac group 14 of the periodic table.
Diamond is used in the manufacture of filaments made of tungstens used for light bulbs.
Graphite is mostly used for refractory battery steel expanded graphite brake linings foundry facings and lubricants.
Diamond dust is used to make saw blades and drill bits that can cut through just about anything.
Graphite while also composed of carbon has an entirely different crystal structure and.
Structure of graphite and uses structure.
Many are considering future applications in the field of medical and aerospace industry.
This derivative of graphite is further used in making lightweight and strong sports equipment.
Graphite has a high melting point similar to that of diamond.
These sheets are said to be 100 times stronger and 10 times lighter than steel.
Graphite is a stable form of naturally occurring carbon also known as plumbago blacklead or mineral carbon.
It is soft and slippery and its hardness is less than one on the mohs scale.
It has a soft slippery feel and is used in pencils and as a dry lubricant for things like locks.
Carbon is an element.
You can think of graphite rather.
In this video of all about diamond in hindi we will learn diamond structure uses of.
It is used in the making of jewelry.
You have to break the covalent bonding throughout the whole structure.